| . |  |
. |
According to the classic rules of physics, substances melt at a lower temperature when their sizes decrease. But scientists at Indiana University Bloomington have found that at least one substance, gallium, breaks the rules, remaining stable as a solid at temperatures as much as 400 degrees Fahrenheit above the element's normal melting point. Their report will be published in an upcoming issue of Physical Review Letters. The discovery gives hope to some nanotechnologists and "nanocomputer" engineers, who have been worried that components will behave unpredictably at smaller sizes, possibly even melting at room temperature. "We expect this finding will interest nanotechnologists and the manufacturers of tomorrow's computers," said chemist Martin Jarrold, who led the National Science Foundation-funded research. "But we also believe chemists will find this phenomenon exciting -- it totally confounds their expectations." Jarrold and his collaborators showed that clusters of a few gallium atoms remain solid rather than becoming liquid near the element's normal melting point, 86 F. Just as surprisingly, the researchers showed that the tiny gallium clusters are actually more stable as solids when composed of 17, 39 and 40 atoms than a gallium slab containing trillions of atoms. Jarrold decided to test the stability of a substance that is especially sensitive to temperature changes near room temperature. Fitting that description is the metallic element gallium, which has an unusually low melting point. Placing a warm fingertip on a cube of gallium will cause it to melt. The researchers constructed a special device to shoot tiny gallium particles containing just a few atoms into gaseous helium. Collisions with the helium atoms broke the gallium clusters into small pieces. Two mass spectrometers measured the size of the intact and broken gallium clusters. By measuring the energy needed to break the clusters into pieces, the researchers were able to determine whether the clusters were in liquid or solid states. Jarrold and his team observed that gallium clusters with 39 and 40 atoms melt at around 531 F. Gallium clusters containing 17 atoms didn't melt at all across the minus 297 to 837 F temperature range the scientists surveyed. Why the gallium clusters retained such stability at high temperature is a mystery. Not all elements or compounds are likely to behave as gallium does. Using the same method, Jarrold previously learned that particles of sodium chloride -- table salt -- obey the classical rules of physics. Small salt particles with just a few atoms melt at low temperatures. Despite its potential implications for industry, Jarrold said his discovery and his general interest in atomic and molecular clusters, which began at Bell Laboratories, are mainly academic. "I just think it's fascinating to ask how small you can make something before its properties change," Jarrold said. IUB chemists Gary Breaux and Robert Benirschke, Nagoya University (Japan) chemist Toshiki Sugai, and Intel Corporation scientist Brian Kinnear also contributed to the report. All of the report's contributing authors were at IUB when the study was completed. Related Links Indiana University SpaceDaily Search SpaceDaily Subscribe To SpaceDaily Express 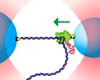 Stanford - Nov 27, 2003
Stanford - Nov 27, 2003When Ralph Waldo Emerson said that nature pardons no mistakes, he wasn't thinking about RNA polymerase (RNAP) - the versatile enzyme that copies genes from DNA onto strands of RNA, which then serve as templates for all of the proteins that make life possible.
|
| ||||||||||
| The content herein, unless otherwise known to be public domain, are Copyright 1995-2016 - Space Media Network. All websites are published in Australia and are solely subject to Australian law and governed by Fair Use principals for news reporting and research purposes. AFP, UPI and IANS news wire stories are copyright Agence France-Presse, United Press International and Indo-Asia News Service. ESA news reports are copyright European Space Agency. All NASA sourced material is public domain. Additional copyrights may apply in whole or part to other bona fide parties. Advertising does not imply endorsement, agreement or approval of any opinions, statements or information provided by Space Media Network on any Web page published or hosted by Space Media Network. Privacy Statement All images and articles appearing on Space Media Network have been edited or digitally altered in some way. Any requests to remove copyright material will be acted upon in a timely and appropriate manner. Any attempt to extort money from Space Media Network will be ignored and reported to Australian Law Enforcement Agencies as a potential case of financial fraud involving the use of a telephonic carriage device or postal service. |